Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Functional Architecture of Personalized & Precision Medicine (PPM) Driven by Innovative Strategies to Secure the Clinical Impact and Implementation to Cancer Management: Brief Comments
*Corresponding author:Sergey Suchkov, Department of Endocrinology, Institute for Global Health, Astrakhan State Medical University, Russia.
Received: September 26, 2022; Published:October 18, 2022
DOI: 10.34297/AJBSR.2022.17.002338
Abstract
The medicine of the XXI century is Personalized & Precision Medicine (PPM), by protecting and preserving human health throughout the life. Creating and maintaining a high level of public health and thus the wellness is a priority in the health sector. In this regard, an upgraded model of healthcare service, which includes the philosophy, principles and armamentarium of PPM and aimed at identifying the disorder at its early (subclinical) stage, is being created and set up. PPM focuses on predictive and preventive measures that contribute to the development of individualized strategies for managing a healthy lifestyle that stabilize morbidity rates and can help to im-prove the working capacity of the population. Against the background of the development of PPM, there was an explosion and the evolution of the concept of biomarkers (specific bioindicators), whose combinations have been being studied, and their effectiveness and efficacy as working re-sources were being evaluated.
Keywords: Personalized; Precision Medicine; Cancer Management; Biomarkers; Nosology; Morbidity; Mortality; Disabling Rates; Biology; clinical decision support (CDS); TraMed; Theragnostic Nanoparticles (TNP); Single-Photon Emission Computed Tomography (SPECT); Positron Emission Tomography (PET); Computed Tomography (CT); Active Pharmaceutical Ingredient (API); Magnetic Resonance Imaging (MRI); Near Infra-Red (NIFR); Precision Therapeutic Approaches (PTA); Artificial Intelligence (AI); Molecular Imaging (MI); High Performance Liquid Chromatography (HPLC)
Introduction
Over the course of history, healthcare and thus healthcare philosophy have been focused predominantly on efforts to probe the already diseased individual by focusing down on a type of disorder (nosology) rather than on health or so-called pre-illness conditions. Much less effort has been placed on keeping individuals from developing disorders in the first place. PPM is expected to transform this situation giving healthcare professionals of tomorrow much more reliable control over morbidity, mortality and disabling rates, and significantly optimize the cost and efficacy of treatment for those who have fallen ill and already diseased or are still persons-at-risk. PPM (Figure 1) is a name for the grand new paradigm in healthcare management being based first on prevention, pre-clinical detection of the illness, and delivery of drugs to target tis-sues with exceptional levels of precision [1].
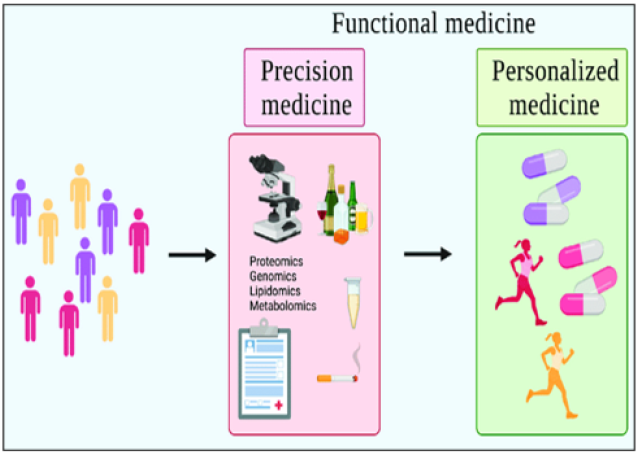
Figure 1: schematic model of Precision and personalized medicine (PPM). Precision vs. personalized vs. functional medicine. Precision medicine identifies differences in individuals, categorizing based on environmental, biological, and psychosocial factors. Personalized medicine takes these differences and implements preventions/treatments tailored to the individual. Functional medicine is an overarching term that seeks to encompass both precision and personalized medicine.
Scientific and Workable Platforms to Secure the PPM Progress
The Grand Challenge of PPM is to forecast, predict, prevent, and treat exceptionally, is rooted in the most recent developments in systems biology, bioinformatics, nanomedicine, and translational medicine. Each of those sciences is a separate niche activity that we hope to see unified to create a new healthcare continuum with great potential future impact on quality-of-life outcomes [1,2]..
Systems biology and OMICS technologies: PPM as being the Grand Challenge to forecast, to predict and to prevent is rooted in a big and a new science generated by the achievements of Systems Biology and Translational Medicine (TriMed), whilst integrating and consolidating platforms of Fundamental Sciences and Newer OMICs Technologies, which are being implemented as The Newest ENTITIES into the daily medical practice to secure Clinical, Subclinical & Predictive healthcare-related manipulations of the next-step generation [3].
The core information resources of systems biology are the “omics” sciences (Figure 2A, B).
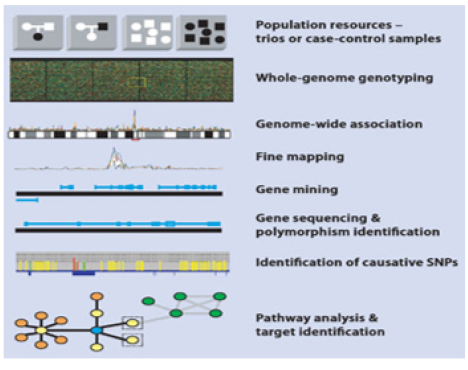
To really understand PPM and PPO we would have to understand the various fields of translational applications that provide the tools to exploit and practice PPM, and genomics tools, in particular! (Figure 3A, B) and are two techniques in Personalized Genomics technologies-related portfolio. Generally, they provide information about the genome of an individual.
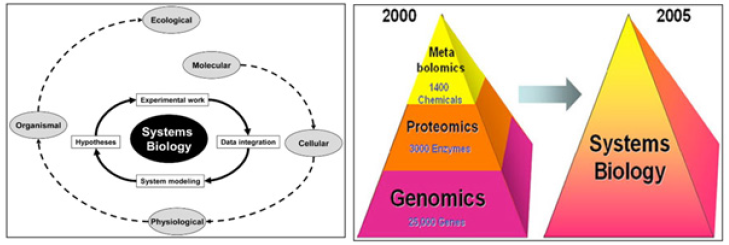
Figure 2A & 2B: Systems Biology. Systems biology incorporates the whole workflow from experimental design and data management through data acquisition, pro-cessing and modelling to visualization and interpretation of the experimental findings. The goal of systems biology is to discover new emergent properties to better understand the entirety of processes that happen in a biological system. Some of the areas of study are listed below, along with the associated OMICS-technologies being integrated into OMICS portfolio.
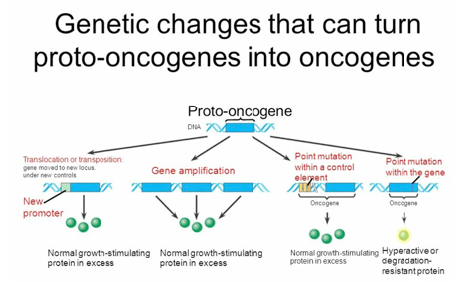
Basically, DNA profiling is the technique, or even approach, widely applicable in PPM- and PPO-related genomic testing for the identification of individuals depending on their genetic makeup [4].
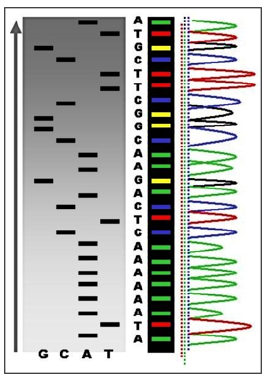
The main difference between DNA profiling and DNA sequencing is their procedure and clinical importance (Figure 4). The human genome holds the clues to diverse diseases and improved quality of life. Pro-vocatively, one can raise a question whether in the genome resides a signature for healthy dis-ease-free life? What would it take to approach wellness of human beings from a health rather than disease perspective? So, Genomics is a set of the unique biomarkers and thus the molecular tools to probe genome for its quality and now even be tested to secure genome profiling (see above).
Genomics probes the general principles of the functional
architecture of genomes. So, genetic testing can provide information
about person’s genes, their products, and chromosomes [5]. And
thus, for screening and monitoring the human health, family future
and the healthy stability of the nation, a set of testing platforms has
been translated into the daily common and cancer-related practice
to secure the fastest development of the technologies to include:
a. Genome se-quenching and
b. Nucleotide polymorphism testing (Figure 5).
Currently, particular attention is being paid to the development of pathological genomics, which allows not only for molecular genetic diagnostics, but is also an important step to deter-mining the intensity of RNA transcription and protein translation in relation to the onset and development of diseases. A clear example of promising developments in this field are results from translational genomics crystallized into DTC-testing aimed at precise and reliable diagnosis that can inform the implementation of disease preventive strategies and preventative manipulations.
Genetic tests can help to:
a. Diagnose disease
b. Identify gene changes that are responsible for an already
diagnosed disease
c. Determine the severity and aggressiveness of cancer
d. Guide doctors in deciding on the best medicine or
treatment to use for certain individuals
e. Identify gene changes that may increase the risk to
develop a disease.
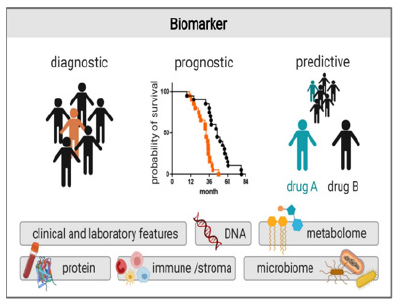
In general, three types of strategic biomarkers are important for PPM- and PPO-related services: Diagnostic, Predictive and Prognostic ones to assess individualized genetic risk prediction tools for a wide array of diseases, and risks, and thus for the national health stability (Figure 6).
A unique example of the applications to unite the abovementioned technologies as applicable to PPO-related cancer monitoring and tumor progression is an approach named as Liquid Biopsy (Figure 7A, B).
Although tumor biopsies continue to be mandatory in cancer diagnosis and classification, several studies have demonstrated that liquid biopsies could be used as a potential tool for the detection of cancer-specific biomarkers. One of the main advantages is that circulating free DNA (cfDNA) provides information about intratumoral heterogeneity, reflecting dynamic changes in tumor burden. This minimally invasive tool has become an accurate and reliable instrument for monitoring cancer genetics as applicable to oncology practice.
Pharmacogenomics-related testing: It is aimed at tailoring drug therapy at a dosage that is most appropriate for an individual patient, with the potential benefits of increasing the clinical efficacy and individualized safety and identifies individual differences in how well or badly people respond to particular drugs [2,6]. Also, pharmacogenetic tests can be used to predict and to target medicines to good re-sponders or to identify whether an individual has an increased risk of a specific adverse drug re-action from a particular medicine (Figure 8A) If genomics is rooted in the development of DNA and RNA sequencing techniques, methods for the identification of individual proteins and antigenic determinants contained there-in play a fundamental role in proteomics.
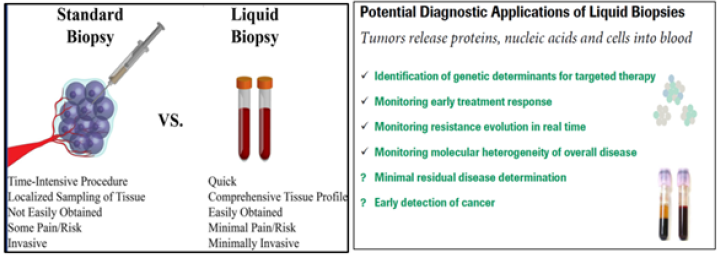
Figure 7A&7B:Standard and liquid biopsies through the View of PPM/PPO. Liquid biopsies can be used to accurately screen for cancer in clinical practice. Traditional biopsies can be difficult to ob-tain but have been the predominant way to diagnose and understand cancer. Liquid biopsies are faster for doctors and easi-er for patients because they take blood samples rather than tissue samples. Liquid biopsy is a minimally invasive technology for detection of biomarkers. Circulating cancer cells or traces of the can-cer’s RNA or DNA in the blood can give clues about which treatments are most likely to work for that patient. Circulating nucleic acids are protected by extracellular micro-vesicles, mainly exosomes. New dedicated methods enable you to enrich and purify from this liquid biopsy: circulating free DNA (cfDNA), circulating small-RNA, circulating tumor cells (CTCs), extracellular mirco-vesicles (including exosomes) containing small-RNA, mRNA and DNA.
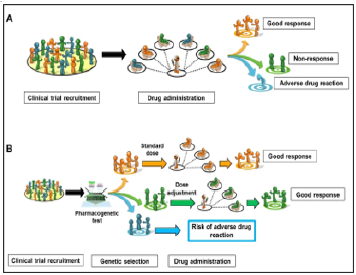
Figure 8A:Comparison of two drug prescribing strategies A: traditional treatment plan without additional genetic testing. B: taking into account the genetic characteristics of patients when pre-scribing a drug, including the genetically determined response of the body to the drug in order to adjust the dose.
Proteomics and metabolic testing: Proteomics relies fundamentally on the use of kits, including immunochemical tests, protein micro-sequencing techniques, High Performance Liquid Chromatography (HPLC) with mass-spectrometry, and on protein microchips for high throughput screening.
A combination of genomic and proteomic/metabolic biomarkers to reflect the complexity of all network pathways in cells at given points in time, are becoming of great significance to be applied in PPM and PPO and need to be translated into the daily practice to predict risks of the chronification and thus of disabling [7].
Molecular imaging: (Figure 8A, B) is defined as the ability to visualize and quantitatively measure the function of biological and cellular processes in vivo and has enormous relevance for patient care: it reveals the clinical biology of the disease process and has the potential to personalize a patient’s care. PPM and molecular imaging will enable us to accelerate and improve cancer management in future medicine. Thanks to the combination of several imaging modalities in the form of the multimodal Molecular Imaging (MI) strategy, a great advance has been made in early diagnosis, in more targeted and personalized therapy, and in the prediction of the results that will be obtained once the anticancer treatment is applied (Figure 9A, B).
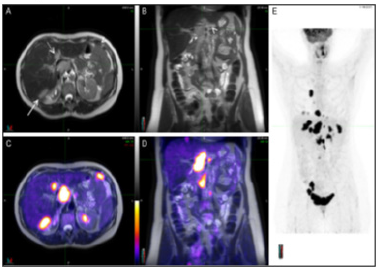
Figure 9A:A typical representative of the PET/MRI molecular imaging benefits in PPM-related PPO cancer care. Staging PET/MRI scan of a 56-year-old woman with ovarian cancer. The MRI images (A and B) show multiple lesions abutting the liver posteriorly (long arrow), involving the porta hepatis (short arrow) and seeding the peritoneum (arrowheads). A round, well defined lesion with same features is also visualized in segment IV of the liver (dotted arrow). On PET/MRI images (C and D) the lesions earlier described, and others not so evident, are depicted by high FDG uptake confirming their malignant nature. Maximum intensity projection of the whole body (E) reveals several lesions both in the chest and abdomen.
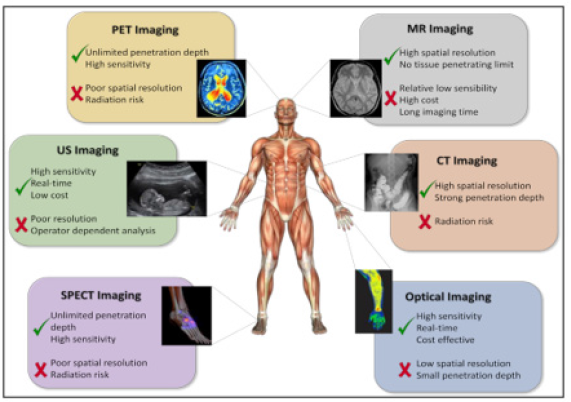
Figure 9B:Molecular imaging techniques. Molecular imaging techniques allow an individualization and optimization of therapy on a patient basis noninvasively. The availability of new hybrid scanners, like PET-computed tomography and PET-MRI allow the combined assessment of changes in morphology and function and are a unique tool for personalized cancer treatment. The development of new specific tracers will enable a more accurate assessment of a therapeutic result.
In this context, magnetic nanoparticles have been positioned as strong candidates for di-agnostic agents as they provide very good imaging performance. Furthermore, thanks to their high versatility, when combined with other molecular agents (for example, fluorescent molecules or radioisotopes), they highlight the advantages of several imaging techniques at the same time. These hybrid nano systems can be also used as multifunctional and/or Theranostics systems as they can provide images of the tumor area while they administer drugs and act as therapeutic agents [8].
The main purpose of imaging in oncology is pre-early and/ or subclinical detection to enable interception if not prevention of full-blown disease, such as the appearance of metastases. Molecular imaging - particularly when combined with liquid biopsy for screening purposes - promises especially early localization of disease for optimum management.
Advances in multimodality imaging, integrating anatomical and functional features of tumors, have allowed guiding clinicians in a prompt decision of the effectiveness of treatments in individual patients, thus reducing therapeutic failures. Indeed, molecular imaging methods allowing in vivo characterization and measurement of biological processes at the cellular and molecular level can detect mechanisms of drug resistance and avoid the use of an ineffective treatment in nonresponding patients. In addition, this approach can identify specific molecular targets, allowing the selection of patients for novel therapies [9].
Molecular imaging will have a major impact on PPO and PPM
by allowing earlier diagnosis, assessing early response to treatment
and by predicting treatment response. It will, hopefully, also
have an impact on drug development by streamlining preclinical
and clinical tests for new drug candidates. The future of clinical
molecular imaging will include:
a. the ability to detect physiologic or molecular changes
which indicate the presence of cancer when it is still at an early and
curable stage,
b. the ability to assess treatment response and adjust
treatment protocols in real time and
c. the ability to streamline cancer drug development
process.
Disease risk assessment could be defined as the systematic evaluation and identification of Risk Factors (Figure 10) responsible for a disease, estimation of risk levels and finding possible ways to counter the onset and progression of a disease or a cancer within the population.
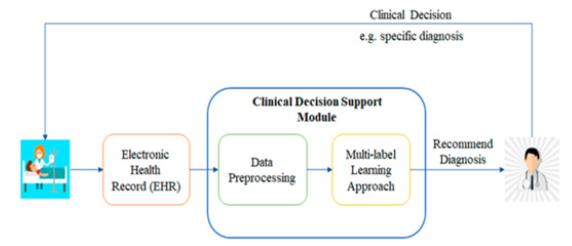
Improved patient (or persons-at-risk) outcomes with the application of the biomarker tests of the next-step generation must consider not only increased survival or quality of life, but also improve Clinical Decision Support (CDS) and make (Figure 11), leading to the avoidance of unnecessary therapy or toxicity captured within the rapid learning system.
Thus, systems biology is helping profoundly to characterize the cooperative networks in cellular and intercellular systems, dynamics, control, and design principles. This information may then be employed to derive new models of practical medicine [1,2].
Bioinformatics (BI) and IT Technologies: The emergence of a large amount of unstructured information because of research is inconvenient. Such information overload is now being resolved by means of two unique technological platforms-BI and Artificial Intelligence (AI) for the analysis of Big Data being harvested from screening, scanning and assessment procedures (Figure 12A, B).
Those two platforms are expected to provide professional communities with the means to mine, integrate, store, process and interpret large quantities of data, as done never. A real bioinformatics challenge has been interpretation of data resulting from the sequencing of the human genome. Furthermore, with the most recent advances in DNA sequencing this field moves rapidly to integrate sequence information from individual patients in real time and to understand the latter through the view of digitalization.
With regard to practical advances gained with bioinformatics, this forms a particular ground for the revolutions in genomics, phenomics and exosomes, and the associated predictive analytics and thus the diagnostic, predictive and prognostic technologies. A significant amount of data harvested from the body sources can be retained in even a single database to demonstrate connections and interrelationships for each body cell type. This has led to the im-pressive results accumulated in the fields of neurology, endocrinology of cardiology and oncology [11].
Thus, the BI-related armamentarium is becoming open and thus able to provide the newest technological support for the PPM health care model being implemented into the daily practice.
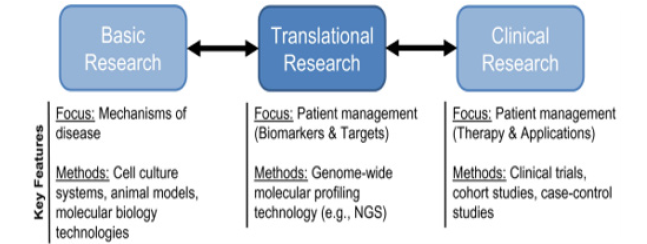
Translational medicine (TraMed): Within the framework of a PPM healthcare model, a special niche is occupied by the re-sources and tools for TraMed (Figure 13).
The global main goal of TraMed is to bring together disciplines, resources, experience, and armamentarium within this niche to prompt laboratory innovations ahead to reach the practice and to thus improve prevention, prophylaxis, diagnosis, treatment, and rehabilitation. TraMed is considered today as interdisciplinary field of scientific and medical endeavor, linked with a deep knowledge of drug development, intellectual property, and regulatory issues, all to define optimal mechanisms for translation of the latest science and healthcare concepts from bench to bedside, and often involving the formation of spin-out biopharma and biotech companies along the way. This is giving rise to a new philosophy and definition of a term of interdisciplinarity and communications to underpin an explosion of healthcare innovations in the very near future to come.
TraMed is also a binary concept that follows “from research to patient and persons-at-risk”, aimed at facilitating and implementing clinical trials involving new therapeutic strategies derived from the latest scientific research, and “from patient to research”, aimed at using clinical feedback to inform and then drive the innovation of even better, more appropriate therapeutic strategies into the clinic [2].
TraMed goal and a unique achievement of XXI-century biomedicine:
Currently, the principal priority of TraMed is to find potential and highly informative biomarkers with their subsequent selection. It is worth noting that the most important achievement of TraMed is the identification of biomarkers pathogenic pathway [12].
As a strategic product of translational applications, biomarkers, that gave impulse to the development of the concept of the precise diagnostic and targeted therapy, provide an opportunity to create tools belonging to the fundamentally new generation [13]. Moreover, discovery and clinical application of biomarkers of the principally next-step generations are expected to play a significant role in reshaping life science research and healthcare biopharma and biotech, thereby profoundly influencing the detection, monitoring and curative effects in a broad scope of disorders including cancer in particular (Figure 14A, B).
Figure 14A,B:Biomarkers as unique achievement of biomedicine. A number of applications of PPM (e.g., a growing number of genomic markers of efficacy, adverse events and dosing of therapeutics) have been proposed and have contributed to oncology practice and healthcare as a whole at many points in an individual’s (including patient’s and person-at-risk’) lifespan. So, we might stress that the translation of PPM and PPO into clinical care and health policy has lagged behind the pace of basic science discoveries.
According to the trend-affiliated data, future challenges illustrating the biomarkers world would also include the development of combinatorial mathematical algorithms to handle simultaneous analysis of many parameters (perhaps up to thousands even) illustrating the functional architecture of the interactome-based networks to aid the diagnosis be confirmed. And a comprehensive understanding of the relevance and validity of each (regardless to being simple or combinatorial ones) biomarker will be very important to efficiently diagnose the cancer condition and provide appropriate direction in the multiple therapeutic alternatives.
Precision Therapeutic Approaches: Whilst the above are a
platform for the creation of a comprehensive personalized medicine
approach to disease prediction and prevention, what about
prospects for comprehensive Precision Therapeutic Approaches
(PTAs) for chronic disease treatment. We define such PTAs for
chronic disease treatment as having the following basic elements:
a. Identification of disease target cells in situ – potentially
achievable through the application of advanced diagnostic imaging
techniques such as Magnetic Resonance Imaging (MRI), Near Infra-
Red (NIFR) fluorescence or resonance Raman, used in appropriate
combination with imaging nanoparticles (or Theranostics
nanoparticles [drug-delivery combined with imaging]).
b. Guidance of theragnostic nanoparticles to disease target
cells – potentially made possible by the exogenous application
of a tissue irradiation technique, such as image-guided focused
ultrasound (IgFUS), to direct Theragnostic Nanoparticles (TNPs)
to accumulate in disease target cells to clear disease state(s) there.
Target-cell receptor specific targeting ligands may also be attached
to TNPs to facilitate this process.
c. Confirmation of therapeutic effects – potentially made
possible by the accumulation of TNPs into disease target cells.
Diseased cells should be visible for as long as cells survive and/or
the disease state continues.
Such PTAs for disease treatment may be described as true image-guided approaches for treatment beginning with detection based on the use of bespoke imaging nanoparticles (or TNPs) for use in combination with advanced diagnostic imaging modalities such as MRI, NIFR and resonance Raman (as above) [but also Computed Tomography (CT) and nuclear medicine imaging techniques such as Positron Emission Tomography (PET) or Single-Photon Emission Computed Tomography (SPECT)]. Once disease target cells are visible to advanced diagnostic analysis, then personalized medicine (as described above) offers the detailed background information necessary to select the best choice of Active Pharmaceutical Ingredient (API) for highly controlled delivery to diseased cells in vivo using bespoke TNPs under the influence of tissue irradiation (as noted above), giving rise to target-focused drug delivery, then highly effective treatment of a given patient under examination. Currently, the detection of disease target cells in situ has still to be perfected in the case of virtually all chronic diseases, so the advent of comprehensive PTAs for the treatment of chronic diseases is currently a future opportunity rather than a present reality, but not for long we would venture to hope [3, 10, 13].
Must-have key to unlock the Personalized & Precision Medicine (PPM) and Oncology (PPO)
Development trends of fundamentally new type of practical health care (personalized medicine) dictate new healthcare requirements. Meanwhile, Precision Medicine and Personalized Medicine have so much overlap that they are often used interchangeably in practice whilst illustrating an integrative term of PP and PPO. So, PPM and PPO are a goal of healthcare, in which evidence-based clinical diagnostic and treatment decisions are informed by each person’s unique clinical, basics (“OMICS”) and environmental (exosomes-related) (Figure 15) information [2,14].
Figure 15:Using information about the impact of the microenvironment on a person for a personalized approach h.
Today two global objectives of PPM and PPO are:
a. screening for subclinical imbalances and defects with a
pre-selection of suitable targets to secure the next steps of PPM
and/or PPO protocol, i.e., prevention, therapy & rehabilitation
b. repair of the imbalances and defects mentioned to restore
the function and to thus prevent the clinical illness and/or to get
defects and imbalances repaired.
More than 11 million people are diagnosed with cancer every year. Biomarkers are there-fore becoming invaluable tools for cancer detection, diagnosis, prognosis, and treatment selection. These can also be used to predict the malignancy, to identify the subclinical stage, to localize the tumor and determine its stage, subtype, and response to therapy. Identification of such signature in surrounding cells or at more distal and easily sampled sites of the body can also influence the management of cancer.
Diagnostic, predictive, and prognostic biomarkers are
quantifiable traits that help oncologists at the first interaction with
the suspected patients or persons-at-risk. Those aid in:
a. identifying who is at risk.
b. diagnose at the pre-early stage.
c. select the best preventive and canonical therapeutic
treatment modality, and
d. monitor response to the manipulations.
Those biomarkers mentioned exist in different forms covering
a broad scope of:
a. cytogenetic and cytokinetic markers.
b. genetic and epigenetic biomarkers.
c. cells (CTCs, T-regulatory cells, cancer stem cells/CTCs,
etc) as biomarkers
d. viral biomarkers.
e. cancer antigens.
f. heat shock proteins/HSPs.
g. mitochondrial markers.
h. metabolic biomarkers.
i. therapeutic biomarkers, etc.
A major challenge in cancer diagnosis is to establish the exact relationship between cancer biomarkers and the clinical pathology, as well as to be able to non-invasively detect tumors at the pre-early (often subclinical) stage.
To achieve PPM-based treatment for cancer, a practitioner of the future would need bi-markers for securing predictive analytics and prognostic efficacy, for determining prognosis, predicting response to therapy, and predicting severe toxicity related to treatment. Biomarkers are, therefore, an objective measure or evaluation of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention (Figure 16A, B).
Step by step on the way of a global challenge-to save our lives and to secure wellness
“Being human means to be a fighter!” - wrote Johann W. Goethe.
Due to the increase in the occurrence of pathologies of unknown
cause, to preserve and prolong life, it is necessary to take care
of health as early as possible - even before the bright disease
manifestations. Therefore, a future challenge in the cancer research
will be the discovery of novel biomarkers to get PPM and PPO fields
re-armed! For instance, the biomarkers of the future would have to
be used for:
a. screening the general population or individuals at risk
(panels of screening and predisposition biomarkers)
b. the detection of the presence of a particular type of cancer
(panels of diagnostic and prognostic biomarkers)
c. monitoring the progression of the disease, and predicting
the tumor’s outcome (panes of prognostic biomarkers)
d. understanding whether a patient will benefit from a
specific drug treatment (panels of predictive biomarkers)
e. evaluating the drug’s efficacy and optimizing the
treatment, providing the tool to tailor treatment for individual
cancer patients or persons-at-risk (panels of pharmacodynamics
biomarkers).
Thus, policy formation in the field of individual health promotion and protection is one of the priority tasks of national healthcare systems. To achieve the goals of value-based healthcare and the implementation of a PPM healthcare model, it is necessary to combine the assets of the latest advances in basic science with clinical medicine, followed by the introduction and promotion of translational capabilities.
Conclusion
Each unit of PPM and PPO is such an independent qualified segment, as a separate specific brick» of a multidisciplinary functional system. Consequently, PPM-model can permanently work only with interaction between all segments. Creating a damage-proof base, these bricks piece together into unified whole, and a completely new technological model can be created, working for the benefit of society.
As you might see from the above-mentioned, PPM has drastically changed and is keeping on changing the landscape of healthcare. In this sense, due to our viewpoint, all healthcare professionals of the future should be educated to deliver patient-centric care as members of interdisciplinary teams, emphasizing evidence-based practice, quality improvement approaches and bioinformatics.
Based on an interdisciplinary basis, PPM and PPO are developing and producing scientific yield, that can be effectively used in daily clinical practice in the future. No less important is that such a multi-disciplinary alliance should be properly and correctly structured and adopted under real real-world environment.
It is also important to remember that regardless of the diagnostic tools used; ultimately a patient’s treatment course will be decided by their physician (clinician). And thus, greater collaboration between clinician and patient (or person-at-risk) would replace the traditional clinician-dominated dialogue with more effective patient-clinician partnerships. So, the current model “physician-patient” would have to be gradually displaced by a “medical advisor-healthy persons-at-risk” model.
In the conclusion, we want to note that several applications of PPM (e.g., a growing number of genomic markers of efficacy, adverse events and dosing of therapeutics) have been proposed and have contributed to oncology practice and healthcare as a whole at many points in an individual’s (including patient’s and person-atrisk’) lifespan.
The next important step in the direction of the PPM approach should be its early adoption in clinics for future medical interventions! To achieve this goal, PPM needs to rely on datadriven sources of fundamentals, analytical tools and services that draw from the rapidly evolving Hi-Tech areas & healthcare-IT infrastructure. Consequently, in coming year’s next generation biotechnologies will reorient medical practice more towards disease prediction and prevention approaches rather than curing them at later stages of their development and progression, even at wider population level(s) for public healthcare system [15].
PPM calls for a transdisciplinary approach, and considerations for how best to develop innovation frameworks to support safe and effective deployment of the new enabling diagnostic and therapeutic technologies not to treat but to get cured!!! (Figure 16).
Indeed, the health technologies of the next-step generation and their applications can help strengthen and triangulate the Attendant evidentiary base for PPM whilst securing the ideal health and wellness!!!
So, just keep you on the way to understand that the Grand Change and Challenge to secure our health and wellness are rooted not in Medicine, and not even in science! Just imagine WHERE?! In the upgraded Hi-Tech Culture! To secure the next-step outcome in the Therapeutic Future to secure Prevention, Prophylaxis, Canonical Treatment and Rehabilitation as the New Entity!!
Thus, PPM and PPO represent a paradigm shift in health care that is both maturing. There has been a rapid increase in the availability and use of biomarker-based tests and this growth is expected to continue. Accordingly, these upcoming changes will profoundly change the professional activities of medical communities.
References
- Suchkov SV (2019) Personalized & Precision Medicine as a New Model of the Healthcare Services. V Russian Congress of Laboratory Medicine.
- TA Bodrova, DS Kostyushev, EN Antonova, S Slavin, DA Gnatenko, et al. (2012) Introduction into PPPM as a new paradigm of public health service: an integrative view. EPMA Journal, 3(16): 3-16.
- Yadav BS, Tripathi V (2018) Recent Advances in the System Biology-based Target Identifica-tion and Drug Discovery. Curr Top Med Chem,18(20): 1737-1744.
- Gupta PK (2008) Single-molecule DNA sequencing technologies for future genomics research. Trends Biotechnol, 26(11): 602-11.
- Katsanis SH, Katsanis N (2013) Molecular genetic testing and the future of clinical genomics. Nat Rev Genet, 14(6): 415-26.
- Jonathan S Schildcrout, Yaping Shi, Ioana Danciu, Erica Bowton, Julie R Field et al. (2016) A prognostic model based on readily available clinical data enriched a pre‐emptive pharmacogenetic testing program. J. Clin. Epidemiol, 72: 107–115.
- Looße C, Swieringa F, Heemskerk JWM, Sickmann A, Lorenz C et al. (2018) Platelet proteomics: from discovery to diagnosis. Expert Rev Proteomics, 15(6): 467-476.
- Mezzanotte L, van’t Root M, Karatas H, Goun EA, Löwik CWGM, et al. (2017) In Vivo Molecular Bioluminescence Imaging: New Tools and Applications. Trends Biotechnol, 35(7): 640-652.
- Rowe SP, Pomper MG (2022) Molecular imaging in oncology: Current impact and future direc-tions. CA Cancer J Clin, 72(4):333-352.
- Eleftherios Trivizakis, Georgios Z Papadakis, Ioannis Souglakos, Nikolaos Papanikolaou, Lefteris Koumakis, et al. (2020) Artificial intelligence radiogenomics for advancing precision and effec-tiveness in oncologic care (Review). Int. J. Oncol, 57(1): 43–53.
- Topol EJ (2019) High‐performance medicine: the convergence of human and artificial intelli-gence. Nat. Med, 25(1): 44–56.
- Wang DC, Wang X (2021) Discovery in clinical and translational medicine. Clin Transl Med, 11(10): e568.
- Bravo-Merodio L, Acharjee A, Russ D, Bisht V, Williams JA, et al. (2021) Translational biomarkers in the era of precision medicine. Adv Clin Chem. 102: 191-232.
- Prasad V, Fojo T, Brada M (2016) Precision oncology: origins, optimism, and potential. Lancet Oncol, 17(2): e81-e86.
- Laganà A (2022) The Architecture of a Precision Oncology Platform. Adv Exp Med Biol, 1361: 1-22.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.